PIPELINE
With the ability to safely house a range of therapeutic cell types within the body, our novel platform can address a range of indications, generating better outcomes for patients.
Regenerative therapies for T1D and beyond
Therapeutic islet cells + bio-hybrid organ
Demonstrated long-term survival (over 5 years) of islets in an implanted bio-hybrid organ
Bio-hybrid organ has been shown to be well tolerated with a favorable safety profile
100% insulin independence achieved in all patients in cohort A
Measurable reduction in hypoglycemic events
T1D
Our lead program offers the potential to be a transformational treatment solution for people living with type 1 diabetes (T1D), by reducing or eliminating the need for life-long insulin injections. Our ongoing phase 1/2 multicohort trial is designed to study the safety, tolerability, and efficacy of our bio-hybrid organ in insulin-dependent patients with T1D.
As encouraging patient outcomes continue to emerge from our multicohort trial, we are broadening the scope of our T1D program to reach more patients who can benefit from treatment. Additional cohorts will investigate the use of Evotec’s induced pluripotent stem cell–derived islet-like clusters in combination with our bio-hybrid organ.
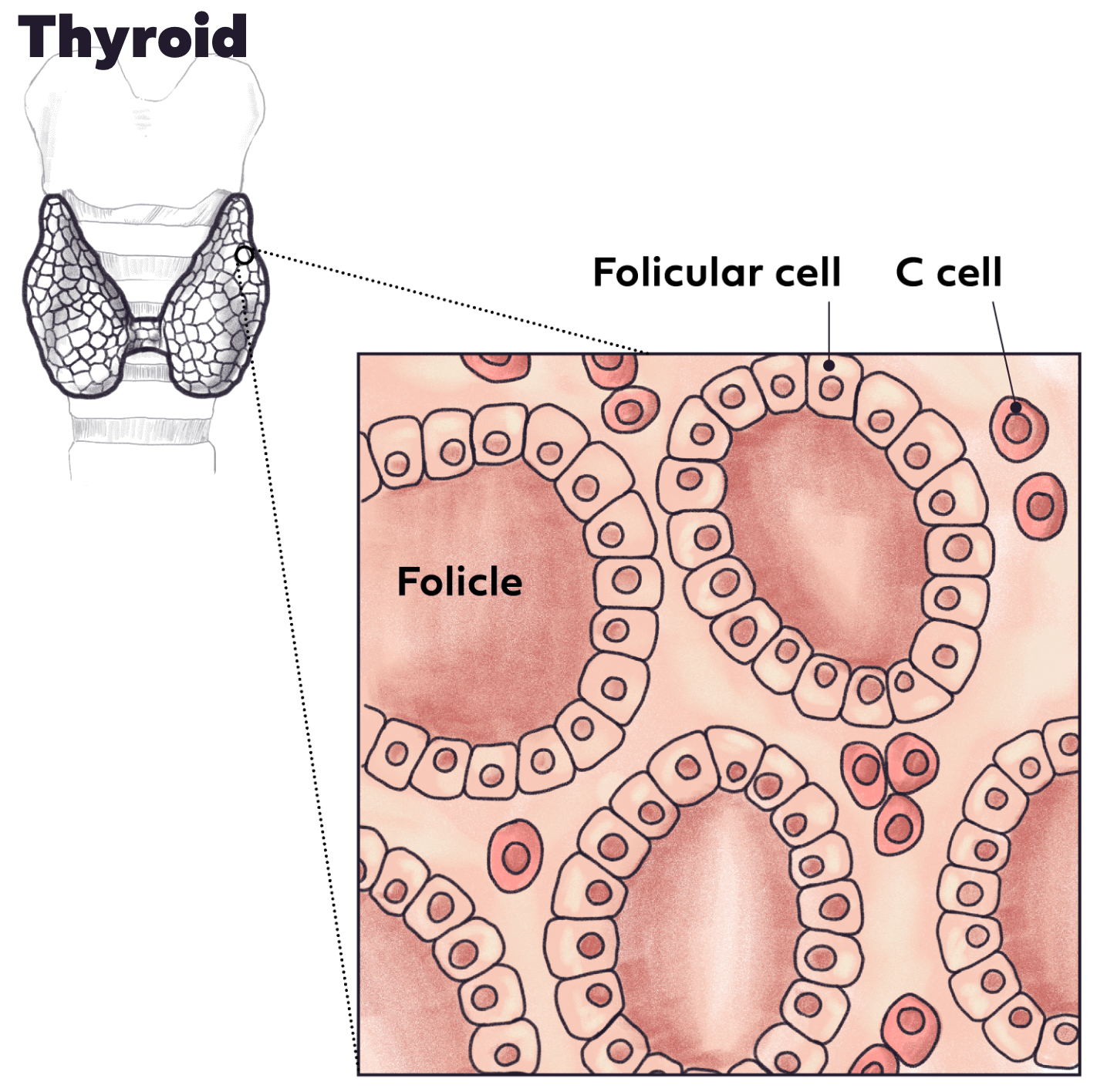
Hypothyroidism
We’re exploring the use of our technology for the treatment of thyroid disease, with an initial focus on hypothyroidism. Using the patient’s own thyroid cells housed within our bio-hybrid organ, we aim to develop an approach that has the potential to reduce or eliminate the need for daily, life-long thyroid medication by restoring the body’s natural feedback loop of thyroid hormones. Our Investigational New Drug (IND) application for this program was cleared by the United States Food and Drug Administration (FDA) in March 2025.