OUR FOCUS
Chronic diseases, including type 1 diabetes (T1D) and hypothyroidism, impact more than 600 million people globally and place a significant economic burden on healthcare systems.1 Despite treatment advances, symptom control remains the standard of care for most of these conditions. Current treatments often require strict dosing schedules, burdensome forms of administration, and constant vigilance.
This is especially true of diseases like T1D, an autoimmune disease that often strikes in childhood and demands lifelong 24/7 monitoring and disease management.
Life, uninterrupted: revolutionizing chronic disease treatment
Placeholder for widget of Bio-Hybrid Organ video
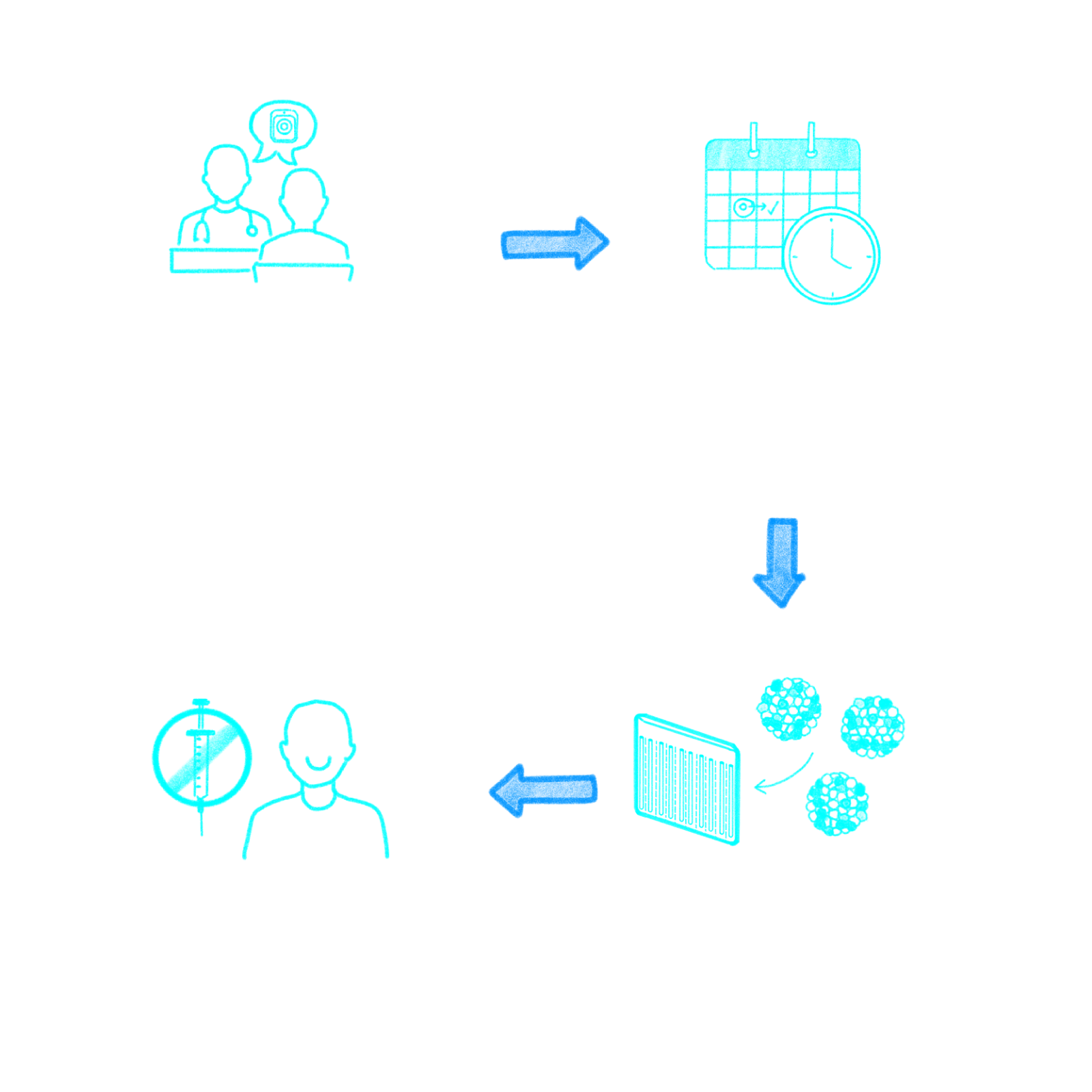
Motivated by our deep understanding of the day-to-day struggles faced by patients and their families, we set out to find a better way. Our therapeutic platform merges our bio-hybrid organ with Evotec’s blood-derived stem cells to correct the underlying deficiencies of hormones, proteins, and other factors caused by T1D, hypothyroidism, and other chronic diseases.
Our bio-hybrid approach to restoring the body’s function
Built with unparalleled medical technologies, our approach to treating T1D and other chronic diseases offers the potential for a safe and effective alternative to burdensome treatment regimens.
Cell Pouch™ bio-hybrid organ
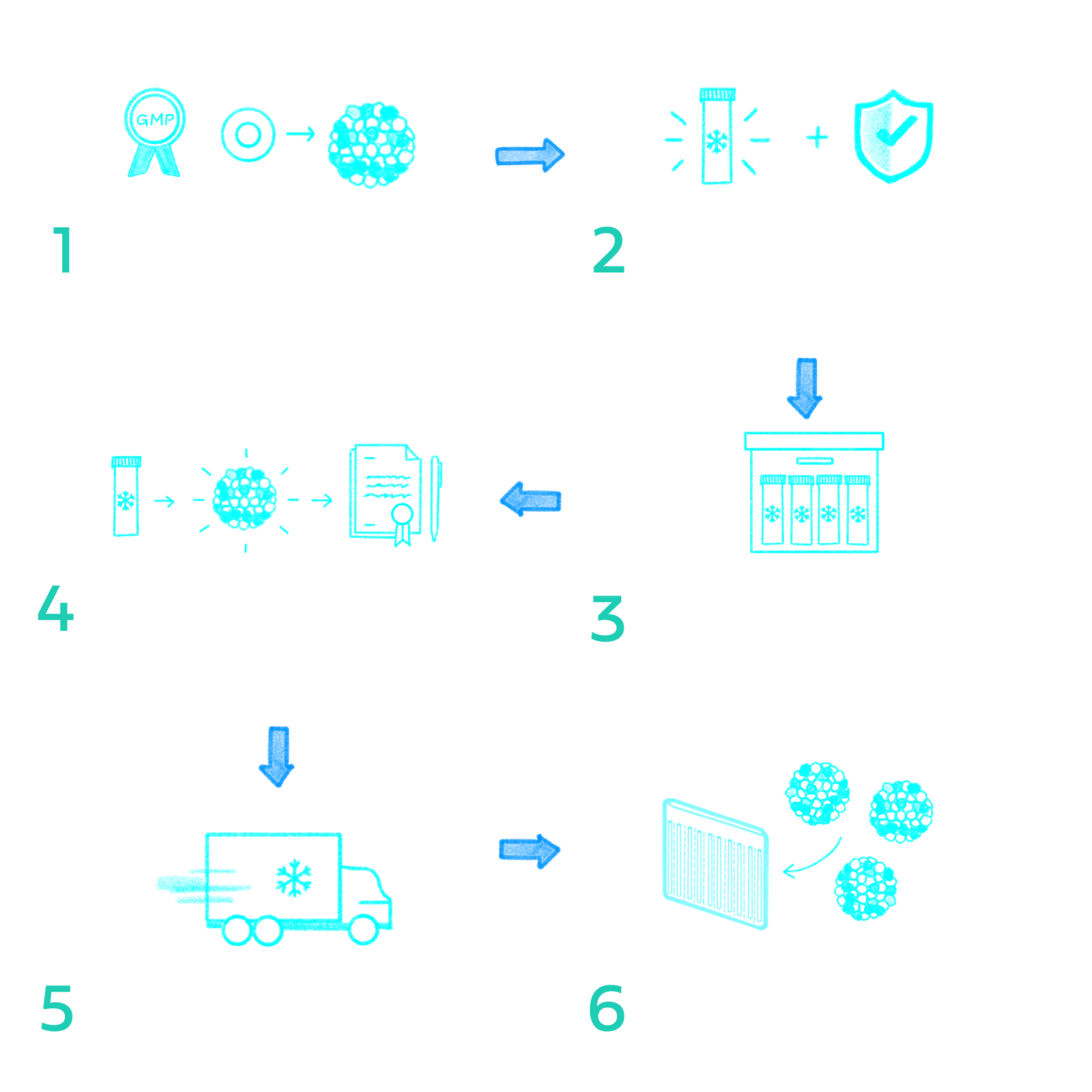
Surgically implanted beneath the skin of the abdomen, our first-in-class scalable bio-hybrid organ is thin, flexible, and credit-card-sized. These qualities allow for seamless integration within the body and the creation of an ideal vascularized tissue environment for the long-term survival and function of the therapeutic cells.
The Cell Pouch bio-hybrid organ can be retrieved from the body after implantation using conventional instruments and methods, if needed, as demonstrated in the ongoing phase 1/2 clinical trial.
Manufactured to the highest regulatory standards from nontoxic, medical-grade, biocompatible materials previously approved by the United States Food and Drug Administration (FDA) for permanent use in the body.
Safe and biocompatible
Demonstrated safety and biocompatibility in multiple preclinical studies. Our bio-hybrid organ has also achieved favorable safety and tolerability in our ongoing phase 1/2 clinical trial with no evidence of fibrosis.
The bio-hybrid organ in combination with transplanted insulin-producing islet clusters, showed efficacy in clinical studies in patients with T1D.
Therapeutic cells: working in partnership with Evotec, we combine our bio-hybrid organ with induced pluripotent stem cells that have been converted from nonembryonic donor-derived cells to create islet-like clusters that closely mimic human pancreatic islet cells. The combination product is set to be the first treatment of its kind to reach clinical testing for T1D.
References: 1. Institute for Health Metrics and Evaluation, Global Burden of Disease Collaborative Network. Global Burden of Disease results. 2021. Accessed November 8, 2024. https://vizhub.healthdata.org/gbd-results/